Our Validated Migration Process
Minimize risk and ensure regulatory compliance during your pharmacovigilance system transition
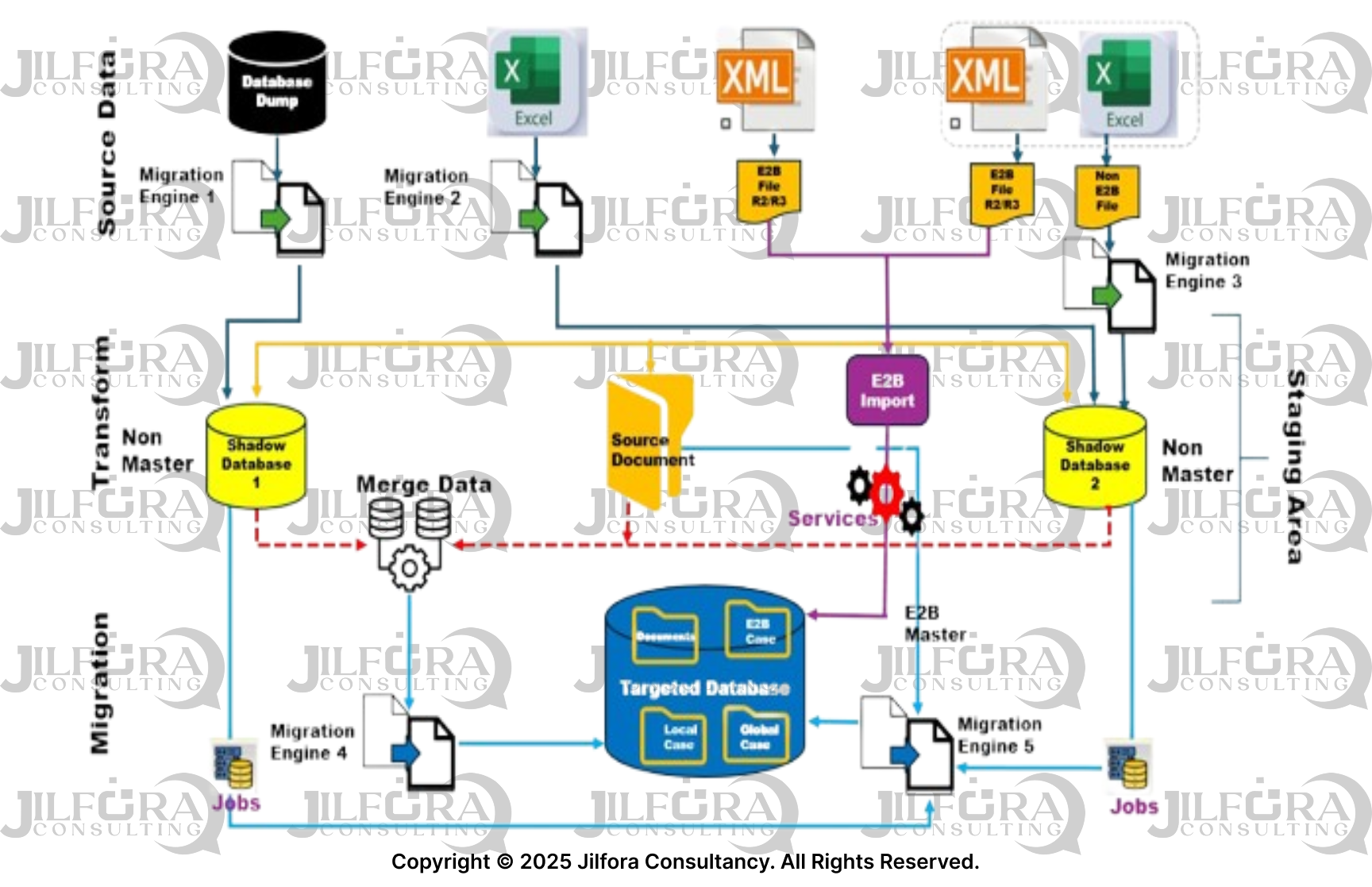
Compliance-Focused Features
Our migration methodology incorporates essential pharmacovigilance requirements
Bidirectional Traceability
Maintain complete audit trails from source to target system with full case history preservation.
Data Integrity
ALCOA+ principles applied throughout migration process with checks for completeness and accuracy.
Global Compliance
Support for regional requirements including EU GVP, FDA 21 CFR Part 11, and Japan PMDA standards.
Historical Data Preservation
Migration of complete case histories including amendments, follow-ups, and regulatory correspondence.
MedDRA/WHO-DD Version Management
Handling of coding changes across different dictionary versions during migration.
Role-Based Access Control
Maintenance of user permissions and access levels during system transition.

