Regulatory Trailblazer: Driving Integrity Through Case Excellence
Proficient in end-to-end case processing, encompassing meticulous data entry, precise product and event encoding, thorough assessment, and critical ICSR validation. Adept at triaging cases and efficiently routing them through complex workflows to ensure regulatory compliance and data integrity
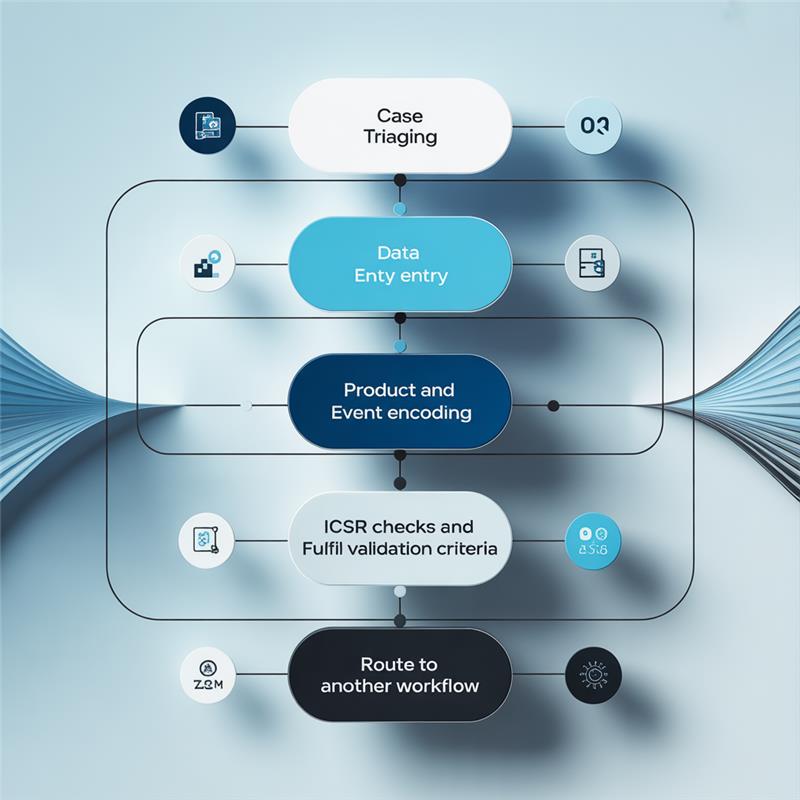
Expert Case Processing Across the PV Lifecycle
Experienced in full-cycle case processing — from data entry and coding to assessment and ICSR validation. Skilled in triaging and routing cases to ensure compliance and data accuracy.
Case Triaging
Initial evaluation and prioritization of reported safety cases based on seriousness, source, and completeness.
Data Entry
Accurate and timely entry of case details into the safety database following regulatory requirements.
Product and Event Encoding
Standardized coding of drug names and adverse events using dictionaries such as MedDRA and WHO Drug.
Medical Assessment
Clinical evaluation of the case by qualified experts to determine causality, seriousness, and expectedness.
ICSR Validation and Quality Checks
Ensuring Individual Case Safety Reports (ICSRs) meet all regulatory criteria for completeness and consistency.
Workflow Routing
Seamless transition of cases to appropriate workflows such as medical review, regulatory reporting, or follow-up, as needed.

